
CONTRAVE Dosing Schedule
The CONTRAVE recommended daily dose is two tablets BID.1 CONTRAVE dosing should be escalated according to the following schedule: 1
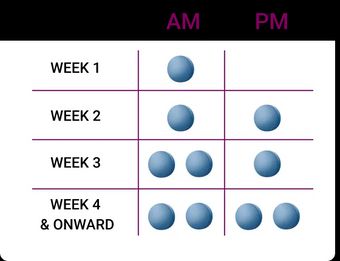
The maximum recommended dose is 32 mg/360 mg per day (two tablets twice daily).1
Dosing Considerations
In order to minimize the risk of seizures, the maximum recommended daily dose should not be exceeded.
Response to therapy should be evaluated after 12 weeks at the maintenance dosage. If a patient has not lost at least 5% of baseline body weight, discontinue CONTRAVE, as it is unlikely that the patient will achieve and sustain clinically meaningful weight loss with continued treatment. 1
Dosage adjustments are required in subjects with hepatic impairment (maximum dose 1 tablet in the morning in mild or moderate hepatic impairment) or renal impairment (maximum dose 2 tablets, one in the morning and one in the evening in moderate or severe renal impairment), and with concomitant use of CYP2B6 inhibitors (maximum dose 2 tablets, one in the morning and one in the evening). All patients with hepatic or renal impairment should be closely monitored for possible adverse effects (e.g., insomnia, dry mouth, seizures) that could indicate high drug or metabolite levels. CONTRAVE should not be taken with a high-fat meal. The tablets should not be cut, chewed, or crushed.1

Blood pressure and pulse should be measured prior to starting therapy with CONTRAVE and should be monitored at regular intervals consistent with usual clinical practice. CONTRAVE should not be given to patients with uncontrolled hypertension and should be used with caution in patients with controlled hypertension prior to treatment. 1

Reference:
- CONTRAVE Product Monograph. Bausch Health Canada, March 2, 2022.
*Please consult the product monograph for complete dosage and administration information.


Find out about ExperienceContrave.ca — one single gateway for easy patient self-enrollment!
